
To ensure the stability of pharmaceutical production and respond to regulations, an AI-based quality prediction and autonomous control system that can monitor and automatically control quality in real time based on process data is required.
In addition, precise data analysis and integrated operation capabilities between systems are required to satisfy complex quality standards such as GMP, QbD, and PAT.
Required expertise: QbD/PAT regulatory response, GMP-based process knowledge, AI quality prediction
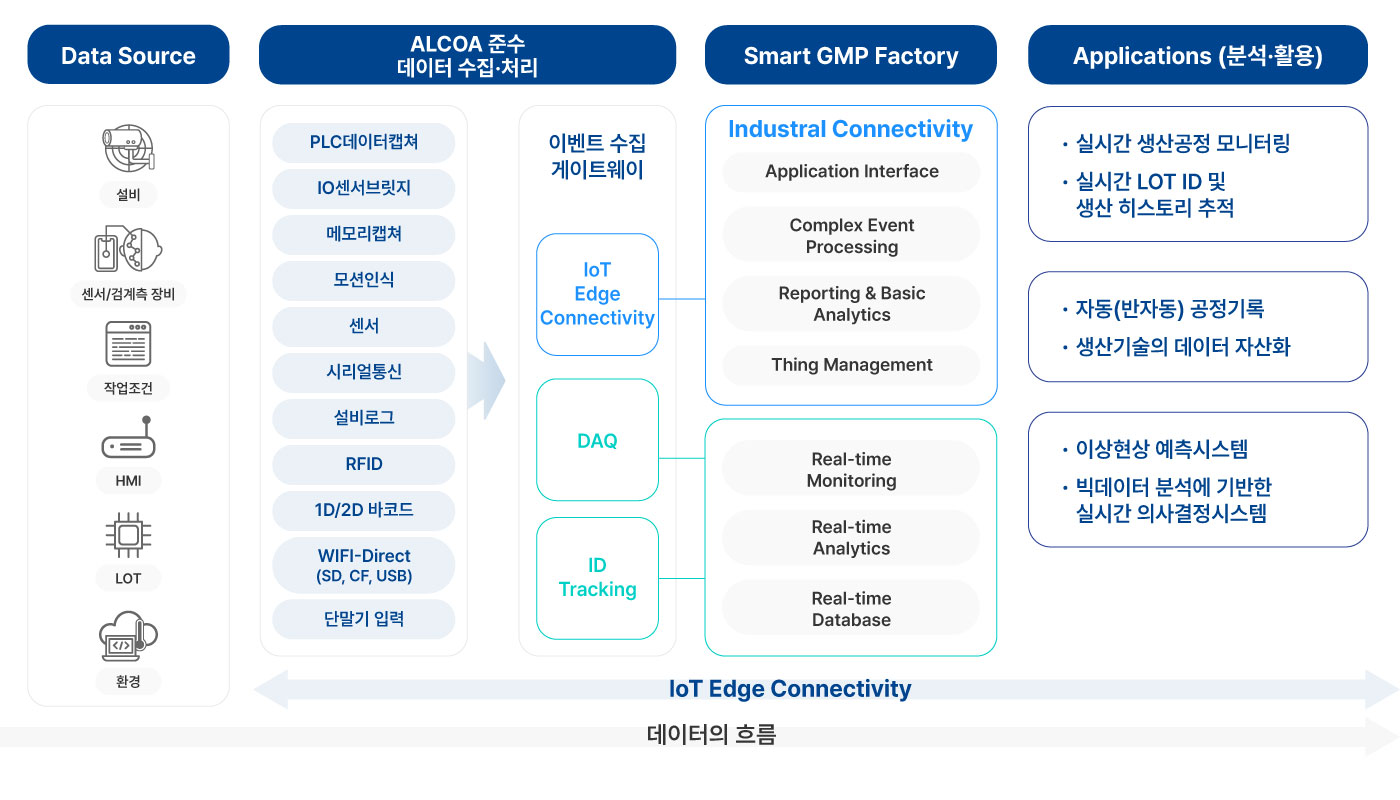
Integrated quality management platform that goes beyond GMP regulatory responses to process optimization and real-time decision-making
Smart GMP Factory is a scalable GMP operation platform that digitizes the entire quality system (PQS), including EBRS, and evolves into an AI-based real-time quality judgment and process optimization solution that can simult
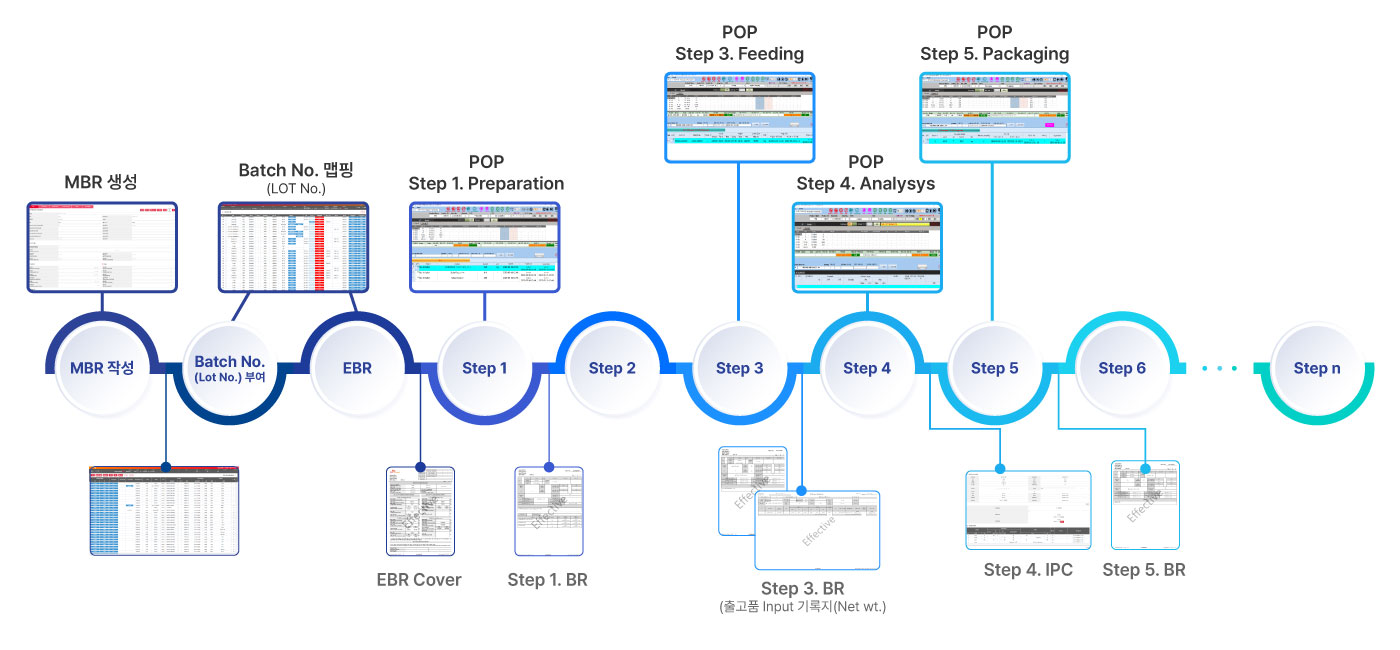
Electronic manufacturing records system
It digitizes the complex and voluminous documentation work that occurs in the pharmaceutical field, enabling systematic recording and management of the entire process from recipe creation to arrange qualification evaluation. It also optimizes productivity and quality control throughout the product life cycle, enabling real-time quality assessment and regulatory response based on accurate records.
REMS is an integrated monitoring system that collects, records, and monitors environmental data such as room temperature, humidity, particle count, and pressure in manufacturing process rooms in real time to ensure compliance with manufacturing quality and GMP standards.
In industries that require a high-quality manufacturing environment, it enables immediate response in the event of environmental abnormalities and supports quick decision-making by workers.

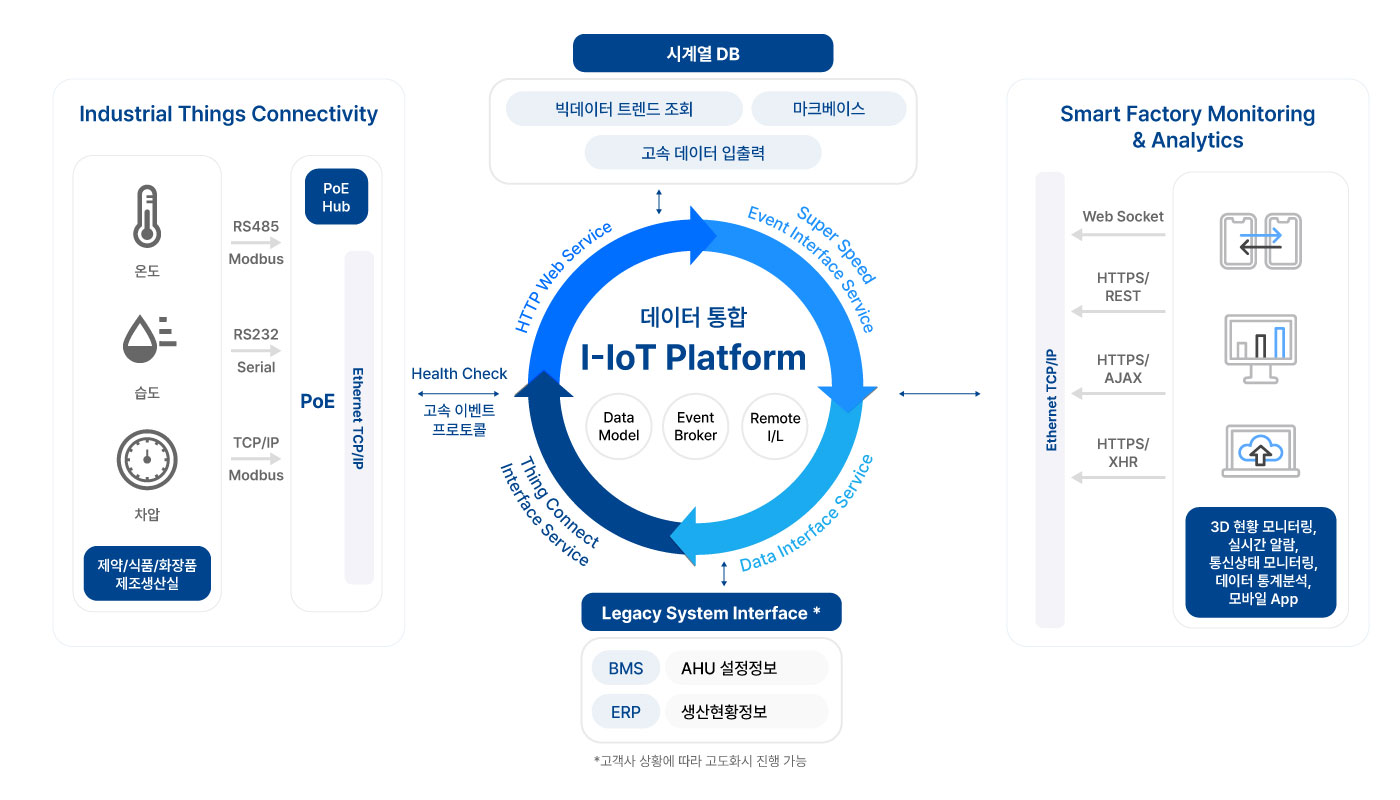
Backup and monitoring solution that standardizes and automates pharmaceutical facility data
It is a system that collects and automatically backs up pharmaceutical facility data that was previously difficult to track in real time, and helps you to systematically manage history. Equipment data is stored in a time-based normalized format and backed up independently of the equipment to optimize lifetime prediction and historical management.


Pain Point
A sophisticated production environment system is needed that allows managers to monitor and control environmental information in real time throughout the entire workplace without having to visit the site, as record management of environmental information is important for pharmaceutical facilities.
Implementation Goals

Using augmented reality (AR), virtual reality (VR), extended reality (XR) equipment and metaverse solutions without space constraints, we built a clean process into a virtual environment and a real-time remote visualization metaverse factory environment in the event of an abnormality.

CCTV footage and equipment data are linked in real time to enable visualization of production site conditions in a metaverse factory environment.
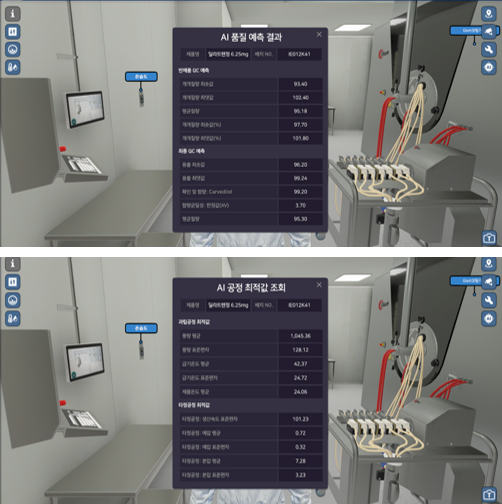
AI models analyze process prediction results, which can be viewed in real time in a metaverse environment, enabling quick quality assessments and responses.
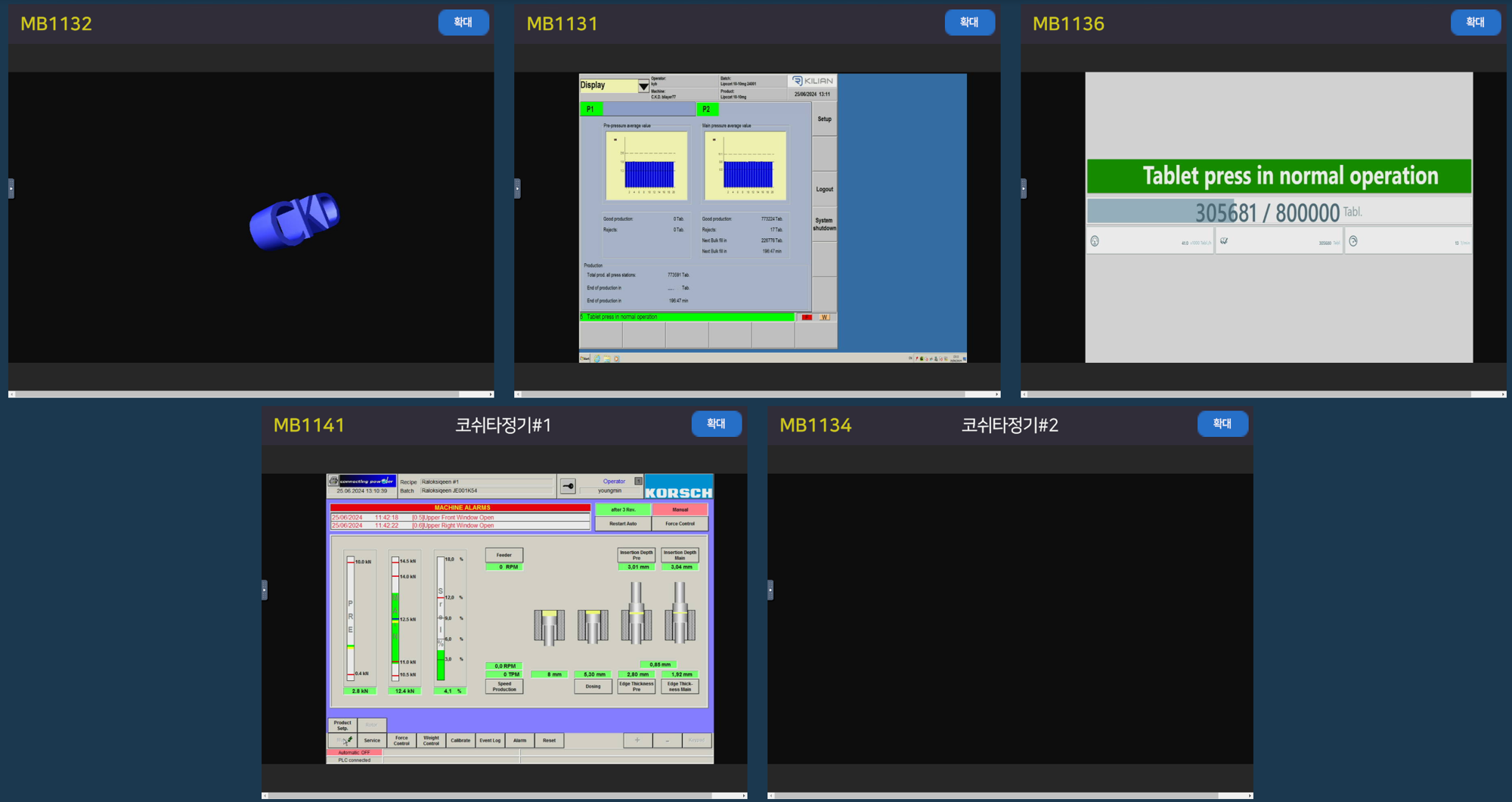
We have implemented a structure that enables real-time remote control of pharmaceutical production equipment operation screens. We offer three types of remote control methods depending on the characteristics of the equipment, taking into consideration operational stability, data security, and real-time control responsiveness.
This enables remote monitoring and control with minimal connections to all equipment.
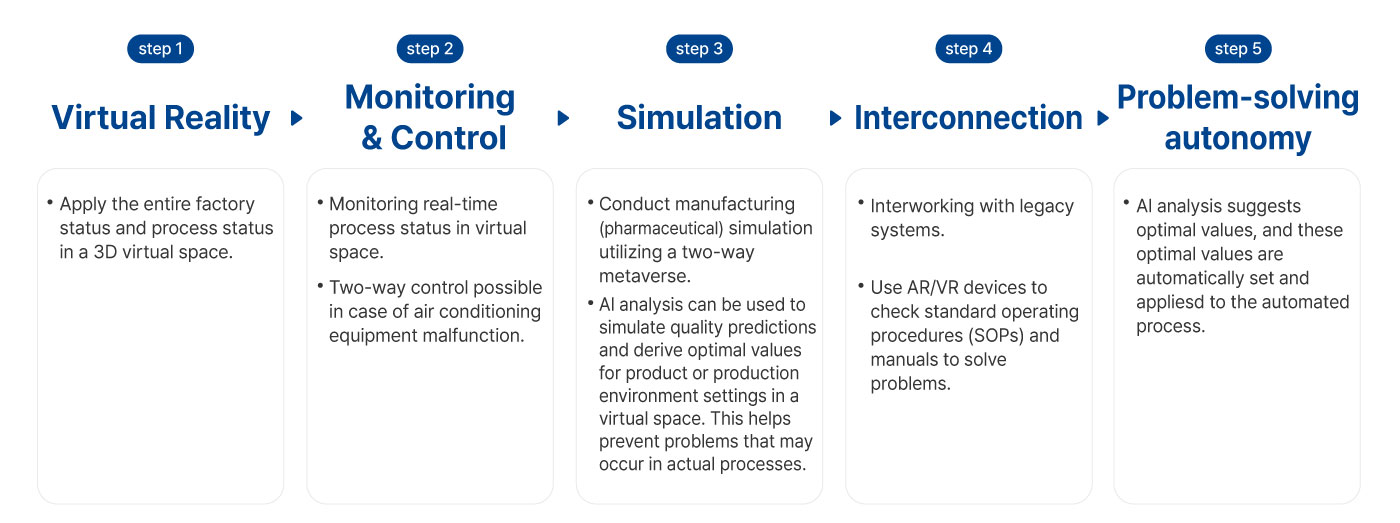
Starting with the construction of a smart factory in 2019,
IMPIX has created best practices optimized for SMEs
through various AX(AI Transformation) projects.